Abstract
Hematopoietic cell transplantation (HCT) can be accomplished with grafts from alternative donors for patients lacking an HLA-matched related or unrelated donor. The optimal choice of an alternative donor stem cell source remains an open question and is influenced by several factors including chronic graft-versus-host disease (cGVHD). Chronic GVHD is a heterogeneous syndrome with major morbidity and adverse effects on quality of life (QoL) among long-term allogeneic HCT survivors. The aim of this study was to analyze the incidence of cGVHD and manifestations associated with severe morbidity and poor functional outcomes among recipients with alternative HCT donors.
Methods: This retrospective study included adults who received a first alternative donor HCT for any diagnosis between 2006 and 2015 in Seattle and subsequently developed cGVHD. The alternative grafts included unrelated 4-6/6-HLA-matched single or double cord blood units (UCB), related HLA-haploidentical bone marrow or mobilized blood stem cells with post-transplant cyclophosphamide (R-HAPLO), and mobilized blood cells from unrelated donors with 1-allele mismatching at an HLA-A, B, C or DR locus at any HLA-typing resolution (1-mMUD). Endpoints were cumulative incidence frequencies (CI) of cGVHD and the CI of manifestations associated with severe morbidity (i.e., grade 2 or 3 keratoconjunctivitis sicca, scleroderma-like skin features, grade 2 or 3 myofasciitis, bronchiolitis obliterans, or esophageal stricture requiring dilation) and the CI of functional outcomes (i.e., return to work/school after the diagnosis of cGVHD, discontinuation of systemic immunosuppressive therapy, and change in KPS). We also compared the 2014 NIH overall severity of cGVHD at initial diagnosis, the incidence of new systemic immunosuppression after first-line therapy for cGVHD, non-relapse mortality and overall survival after cGVHD diagnosis.
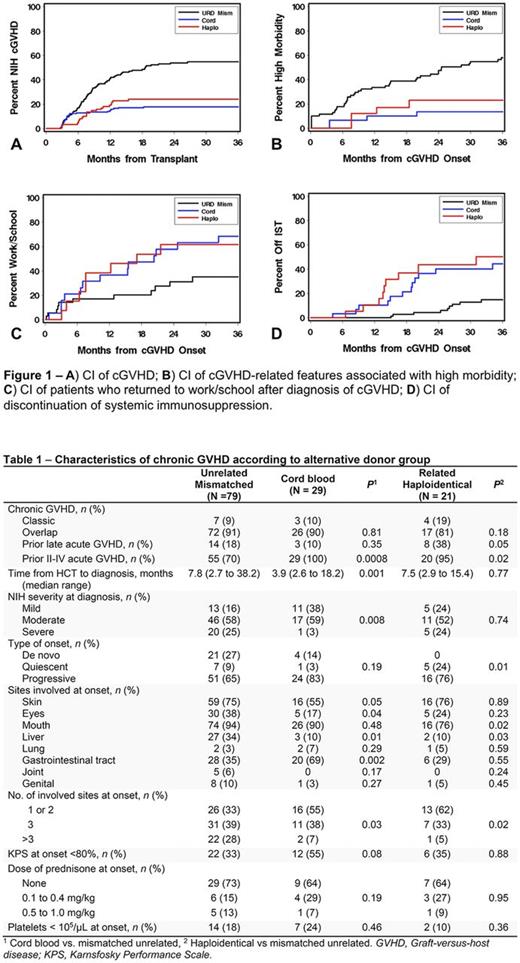
Results: Of 396 alternative donor HCT recipients, 129 developed cGVHD and were included in this study. Chronic GVHD developed in 29 of 163 UCB recipients (3-year CI, 18%), 21 of 88 R-HAPLO recipients (24%) and 79 of 145 1-mMUD recipients (55%) HCT (Fig. 1A). The median follow-up times after onset of cGVHD were 48 (range 4-121) months for UCB, 60 (range <1-123) for R-HAPLO, and 46 (range 4-131) for 1-mMUD. Table 1 displays characteristics of cGVHD according to the alternative donor groups. The CI of any manifestation of severe morbidity at 3 years after a cGVHD diagnosis is shown in Fig. 1B and was significantly lower in UCB (14%) and R-HAPLO (23%) compared to the 1-mMUD group (58%) [hazard ratio (HR) 0.13 (95% Confidence Interval 0.1-0.4), p=<0.0001, and HR 0.31 (0.1-0.7), p=0.007, respectively]. Compared to the 1-mMUD group, a higher proportion of recipients of UCB (68% vs. 35%) returned to work/school at 3 years after cGVHD [HR 2.54 (1.1-5.7), p=0.02], and a similar trend was observed in R-HAPLO (62% vs. 35% [HR 2.38 (1.0-5.9), p=0.06)] (Fig. 1C). The CI of discontinued systemic immunosuppression at 3 years was significantly lower in the 1-mMUD (15%) compared to the UCB (45%) and R-HAPLO (50%) groups [HR 3.96 (1.9-8.4), p=0.0003, and HR 4.93 (2.2-11.1), p=0.0001, respectively], (Fig. 1D). We found trends suggesting improved annualized KPS change from cGVHD onset to 3.5 years in the UCB [+5 (range -12 to +44)] and R-HAPLO [+11 (range -14 to +64)] groups compared to the 1-mmUD group [0 (range -81 to +46)] (p=0.05 and p=0.06, respectively). The proportion of patients in each group requiring change in systemic therapy for control of cGVHD at 3 years after first line treatment was: 17% for UCB, 25% for R-HAPLO and 39% for 1-mMUD, and was significantly lower for UCB compared to the 1-mMUD group [HR 0.30 (0.1-0.8), p=0.01]. Non-relapse mortality and overall survival were similar among the 3 groups.
Conclusions: Compared to 1-mMUD group, UCB and R-HAPLO HCT recipients were less likely to develop cGVHD. Among those with cGVHD, UCB and R-HAPLO HCT recipients were less likely to develop manifestations of severe morbidity, had less protracted cGVHD (a shorter duration of systemic treatment), and higher likelihood of resuming work or school, suggesting better QoL compared to 1-mMUD HCT recipients. Our results will help better counsel patients about alternative HCT donors. A randomized study (NCT01597778) comparing UCB with R-HAPLO HCT is underway to determine which of these donors may be associated with superior outcomes.
Lee: Mallinckrodt: Honoraria; Amgen: Other: One-time advisory board member; Bristol-Myers-Squibb: Other: One-time advisory board member; Kadmon: Other: One-time advisory board member. Martin: Pfizer: Consultancy; Procter and Gamble: Equity Ownership; Incyte: Consultancy; Fresenius, Neovii: Research Funding. Cheng: Gilead Sciences, Inc.: Consultancy. Flowers: Pharmacyclics: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal